Background:
Radiotherapy is a cornerstone of treatment strategies for gynecological malignancies, However, while effective against neoplastic cells, pelvic radiotherapy frequently incurs collateral damage to normal tissues, especially to the bone marrow. This unintended consequence often precipitates thrombocytopenia, a condition characterized by diminished platelet levels, thereby elevating the risk of bleeding and posing hurdles for the continuity of anticancer regimens. Addressing this clinical challenge, hetrombopag, an innovative, orally administered non-peptide thrombopoietin receptor agonist, presents promise. It works by kindling megakaryocyte proliferation and differentiation, thereby enhancing platelet production via the activation of thrombopoietin receptor dependent STAT and MAPK signaling pathways. Previous studies have documented its efficacy in expediting platelet recovery in patients with chemotherapy-induced thrombocytopenia, thereby curtailing the duration of the condition and ensuring the execution of planned chemotherapy cycles. This study aims to investigate the therapeutic effectiveness and safety profile of hetrombopag in the treatment of radiotherapy-induced thrombocytopenia within a real-world context.
Methods:
This retrospective study encompassed gynecological cancer patients who developed thrombocytopenia (platelet counts<100×10 9/L) following radiotherapy and subsequently underwent treatment with hetrombopag from January 2022 to June 2023. The key inclusion criteria included an age of ≥18 years, the development of thrombocytopenia after radiotherapy or a combination of radiotherapy and other anticancer interventions, the administration of hetrombopag as monotherapy or as part of a combined regimen with other platelet-boosting agents, and the availability of follow-up data. The primary efficacy outcome was the proportion of patients exhibiting a platelet response (an elevation in platelet counts to ≥75×10 9/L) within 14 days.
Results:
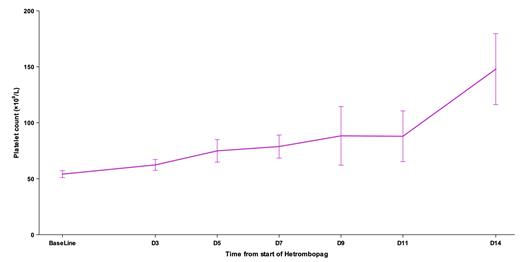
Our cohort consisted of 28 gynecological cancer patients, encompassing 21 (75.0%) cases of cervical cancer, 3 (10.7%) of endometrial cancer, 1 (3.6%) of ovarian cancer, and 3 (10.7%) of other gynecological malignancies. The patients' median age was 58 years (range: 29-76 years), with 50% in clinical stage III/IV. Among them, 19 had received radiotherapy, 8 had undergone concurrent chemoradiotherapy, and 1 had received radiotherapy plus targeted therapy and immunotherapy. The baseline platelet count prior to hetrombopag treatment was 54.1±16.5×10 9/L. Hetrombopag was administered at a dose of 2.5mg/day as monotherapy in 32.1% of patients and combined with recombinant human thrombopoietin or recombinant human interleukin 11 in 67.9% of patients. The observed platelet response rate was 53.6% within 7 days and increased to 75.0% by the 14-day mark. The median time to platelet response was 7 days (95% confidence interval: 5-14). Following the introduction of hetrombopag as monotherapy or part of a combination regimen, we noted a steady uptick in patients' platelet counts. The mean platelet counts on days 3, 5, 7, 9, 11, and 14 were 62.3, 74.9, 78.7, 88.3, 87.9, and 147.9 ×10 9/L, respectively (Figure). Throughout the treatment period, no adverse events related to hetrombopag were reported.
Conclusion:
In conclusion, our findings provide preliminary evidence for the effectiveness and safety of hetrombopag, administered either as monotherapy or in combination, for managing radiotherapy-induced thrombocytopenia. Given the retrospective design of this study, further corroboration is needed through large-scale, prospective, randomized, and controlled clinical trials.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal